Vietnam has launched an important update to its food safety regulation through Government Decree No. 46/2026/NĐ-CP, effective from January 26, 2026.
This new decree replaces the previous Decree 15/2018 and brings modern, streamlined rules for how food products are registered, certified, imported, labeled, and sold across Vietnam.
For foreign investors (FDIs) in the food and beverage industry — whether you’re manufacturing locally, exporting to Vietnam, importing ingredients, or distributing finished products — these changes directly affect your operations.
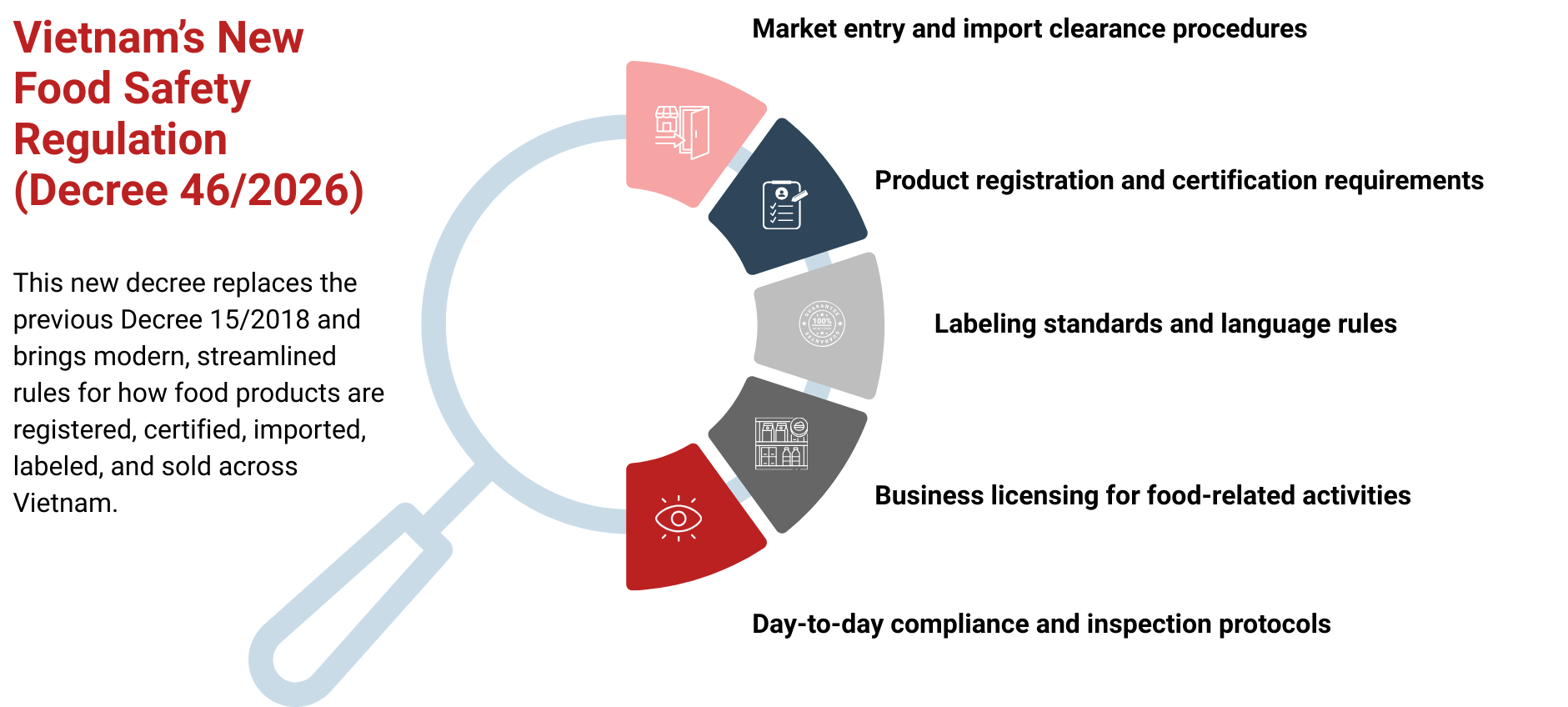
Overview of Decree 46/2026 – A Major Shift in Vietnam’s Food Safety Regulation
Vietnam’s food safety regulation underwent a major update with Government Decree No. 46/2026/NĐ-CP, effective January 26, 2026. This decree replaces Decree 15/2018/NĐ-CP and serves as the key implementing rule under the Law on Food Safety.
It introduces a more systematic, risk-based approach that aligns closer with international standards while strengthening consumer protection.
Key Areas Covered by Decree 46/2026
- Product Standards and Registration Businesses must declare conformity and register food products (including additives) to confirm they meet standards before market entry.
- Production and Business Conditions Stricter hygiene, safety, and facility requirements apply. Eligible operations need a Certificate of Food Safety Compliance.
- Inspection and Traceability Enhanced supervision features risk-based inspections (especially imports), improved record-keeping, and full supply-chain traceability.
- Labeling and Advertising Labels must be accurate and compliant. Health claims — particularly for supplements — face stricter vetting and pre-approval.
- Stakeholder Responsibilities Clearer obligations apply across the supply chain, holding producers, distributors, retailers, and others accountable for safety.
Key Differences and Immediate Impact
Decree 46/2026 imposes tougher rules, especially for imported foods and health supplements. It took effect immediately with no transitional grace period, causing short-term border congestion for agricultural imports in late January 2026 while authorities issued clarifying guidance.
For foreign direct investors (FDIs) in the food and beverage sector, compliance expectations are now significantly higher. Old approvals may no longer suffice without updates to meet the new standards.
The goal is clearer, more transparent oversight to build consumer trust, protect public health, and support Vietnam’s global market integration.
Proactive review and adaptation will help FDIs avoid disruptions and benefit from a more standardized, predictable environment.
Ready to enter Vietnam’s market under the new food safety regulation? Talk to InCorp Vietnam’s experts for fast, compliant product registration and import licensing support.
Market Access and Import Procedures Under the New Food Safety Regulation
One of the earliest and most visible impacts of Decree 46/2026/NĐ-CP for foreign businesses has been on import procedures and market entry. The updated food safety regulation, effective from 26 January 2026, introduced a risk-based inspection system for imported food shipments, fundamentally changing how goods are cleared at Vietnam’s borders.
Under this system, authorities assess shipment risk based on the product category, country of origin, and the importer’s compliance history. High-risk products, such as fresh produce and certain processed foods, as well as shipments from new or untested importers, may be subject to physical inspections, sampling, and laboratory testing. In contrast, importers with a strong compliance track record can expect fewer checks and faster clearance over time.
The new normal for food imports
The risk-based inspection model is now stabilizing and becoming more predictable. Imported food shipments undergo document checks as standard, followed by targeted physical inspections or testing where risk indicators are triggered. While this approach increases scrutiny at the outset, it is designed to reward compliant importers over time with smoother and faster clearance.
What FDIs and exporters should prepare for
Businesses exporting food to Vietnam should strengthen upfront documentation, including certificates of analysis, declarations of conformity, health certificates, and traceability records. Additional buffer time should be built into supply chains, particularly for first-time shipments, as full inspection or testing remains possible until a reliable compliance history is established.
Strategic tip for FDIs
Plan for longer lead times under the new food safety regulation and work closely with experienced local customs brokers or agents familiar with Decree 46/2026/NĐ-CP. Early preparation and strong compliance processes will help minimize delays, reduce costs, and ensure smoother market entry as the system continues to mature.
Overall, the shift reinforces consumer protection and supply-chain transparency. For businesses that adapt early, the new framework creates a more predictable environment that favors compliant and well-prepared importers.
Product Registration and Food Standards Declarations
The updated food safety regulation under Decree 46/2026/NĐ-CP replaces the former self-declaration model in Decree 15/2018 with a stricter, authority-reviewed pre-market approval process. Under this framework, most food products must complete a formal declaration of applied standards before they can be imported, manufactured, or sold in Vietnam.
Who must declare standards
The requirement applies broadly to processed and pre-packaged foods, including food additives, processing aids, food contact materials, health supplements, fortified foods, vitamins and minerals, and special dietary products, even where no health claims are made. Exemptions are limited and generally apply only to raw materials or semi-finished goods not sold directly to consumers, as well as imports used solely for re-export, testing, research, or humanitarian purposes.
How the declaration process works
Businesses submit a declaration dossier, typically via national or provincial online public service portals. Required documents usually include product specifications and safety criteria, accredited laboratory test results, a sample product label, a Certificate of Free Sale or equivalent from the country of origin, and Vietnamese translations of foreign-language documents. The submitting entity bears full responsibility for the accuracy of the information and the ongoing safety of the product.
Review timeline and market entry impact
Authorities review complete dossiers within fifteen working days. If no amendments are requested, the declaration is automatically accepted and published on the official portal. Only after publication may the product be manufactured, imported, or distributed. In practice, this introduces a two to three-week lead time for market entry, even for low-risk products, compared with the near-immediate access allowed under the previous regime.
Special rules for high-risk products and product changes
High-risk categories, such as health supplements, infant formula, and medical foods, continue to require full registration and approval by the Ministry of Health, with closer scrutiny of manufacturing standards. Significant changes to a product’s name, origin, or key ingredients require a new declaration, while minor changes can usually be handled through notification. A single declaration may cover multiple production sites, simplifying compliance for larger FDIs.
What FDIs should do now
FDIs should coordinate early with local entities to prepare declarations before launch, gather supporting documents in advance, and factor the review timeline into commercial plans. Declared standards should be properly recorded and made accessible, particularly for e-commerce sales. Early preparation reduces the risk of delays, rejections, or enforcement action under Vietnam’s strengthened food safety regulation.
Licensing and production compliance for FDI manufacturers
Vietnam’s updated food safety regulation under Decree 46/2026/NĐ-CP tightens licensing and operational requirements for food production facilities, directly affecting FDIs establishing or partnering in manufacturing activities.
Most factories and processing facilities must obtain a Certificate of Food Safety Compliance before operating.
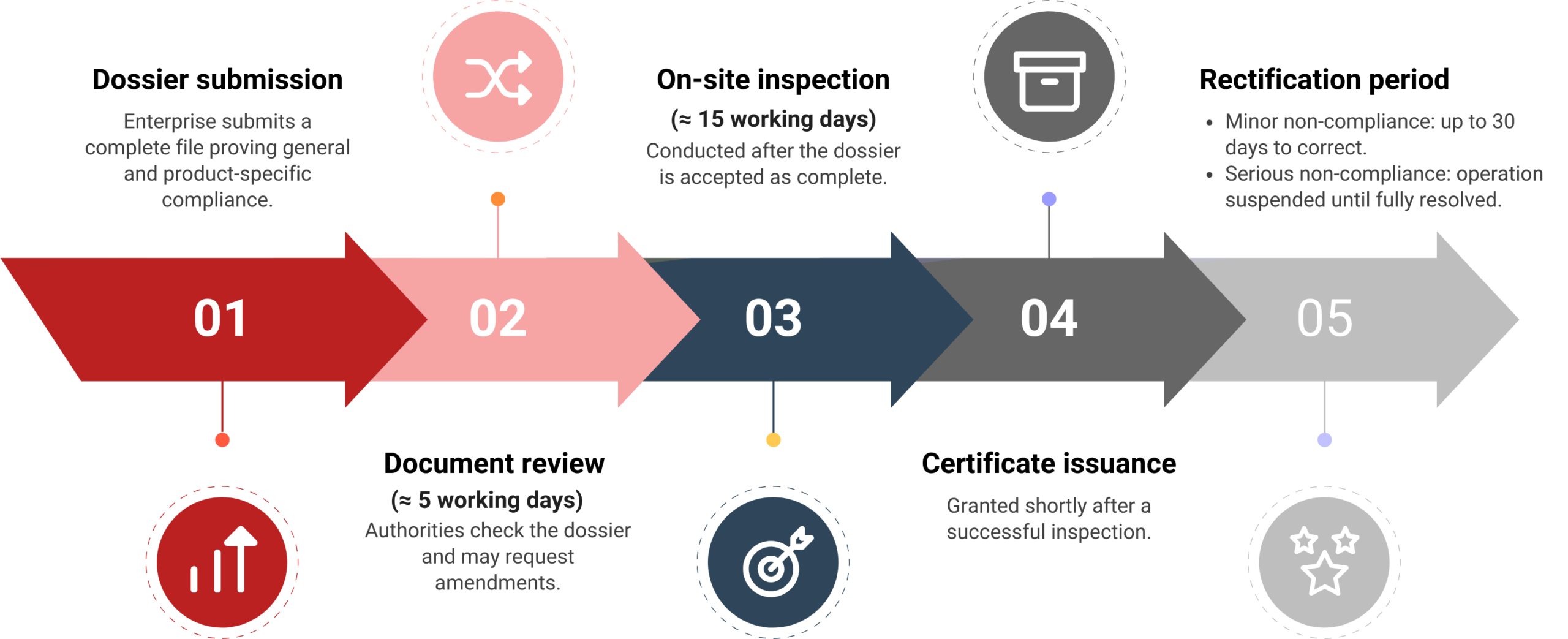
Practical planning: Well-prepared FDIs should allow around 1 month to complete the process.
Certain small-scale or low-risk operations are exempt, such as household producers, mobile vendors, pre-packaged food sellers without processing, or hotel guest kitchens. However, most FDI manufacturing activities do not qualify for exemptions and must still meet full licensing requirements.
For health supplements, compliance is stricter. Production facilities must now implement mandatory GMP standards in line with Ministry of Health guidelines. This includes proper facility layout, documented procedures, batch-level traceability, independent quality control, qualified technical staff, and regular sanitation and monitoring. While these standards align with international practice, some FDIs may need upgrades, training, or additional documentation to comply.
What FDIs should plan for
FDIs should build the certification timeline into project plans, conduct pre-audits to identify gaps early, and treat GMP compliance as a non-negotiable requirement for supplements. Working with local advisors or GMP-certified contract manufacturers can help speed up approvals and reduce regulatory risk.
Overall, the food safety regulation reinforces a clear principle: no certificate means no operation. Early preparation is key to securing licenses quickly and operating smoothly in Vietnam’s regulated food market.
Labeling, Traceability, and Advertising Obligations
Decree 46/2026 also touches upon food labeling and advertising, integrating them into the broader compliance framework. For FDIs, these are not entirely new obligations – Vietnam has had stringent labeling laws for years – but it’s worth highlighting how the new food safety regulation reinforces these areas, as non-compliance here can quickly draw penalties or product seizures.
Advertising and health claims — approval is mandatory
Advertising for health supplements, infant formula, and functional foods now requires prior approval from the Ministry of Health. Misleading claims are prohibited, and supplements must not be promoted as medicines. All health and nutrition statements must stay within registered content. Enforcement is expected to increase, with risks including fines, ad removal, or registration suspension. FDIs should plan approval timelines into every marketing campaign.
Traceability obligations and inspection readiness
Traceability is now a core compliance requirement. Businesses must maintain clear records linking each batch to suppliers, production, and distribution. Decree 46 strengthens inspection expectations, requiring records to be accurate and quickly accessible during audits or recalls.
What FDIs should do now
FDIs should pre-review labels locally, use only approved claims, ensure e-commerce disclosures are visible, adopt digital traceability tools, and treat advertising approval as a fixed compliance step. Early alignment helps reduce risk and protect market access under Vietnam’s food safety regualtion.rate labeling, controlled claims, and strong traceability — reduce risk and position themselves well in Vietnam’s increasingly regulated market.
Enforcement Risks and Compliance Challenges
The rollout of Decree 46/2026 shows that Vietnamese authorities are enforcing the food safety regulation decisively, even at the cost of short-term disruption. Early 2026 border stoppages confirmed that there is no informal grace period. Non-compliance now translates directly into operational and financial risk for FDIs.
First, there is no grandfathering of old approvals. Products approved or self-declared under Decree 15/2018 may need to be updated or reconfirmed under the new framework. During the transition, some applications submitted under the old rules were halted and had to be resubmitted under Decree 46. FDIs should review all existing product registrations and licenses to confirm they remain valid under the new legal basis.
Second, bottlenecks and inconsistent enforcement remain a risk. While initial congestion eased, future guidance or changes in inspection practice could create new adjustment periods. Because inspection powers are more decentralized, procedures may differ between ports or provinces. Strong coordination with customs brokers and logistics partners is essential to identify and resolve issues quickly.
Third, compliance costs are rising. Mandatory GMP for supplements, additional documentation, and increased testing all add to operating expenses and can slow time-to-market. FDIs should budget for these costs as part of market access. The cost of non-compliance, including delayed shipments, spoilage, penalties, or reputational damage, is often far higher.
Finally, penalties and legal exposure are increasing. Violations such as mislabeling, selling unapproved products, or failing declared standards can result in fines, recalls, or suspension. Authorities are expected to expand post-market surveillance, including random sampling of products already on the market.
Risk mitigation for FDIs
To reduce exposure, FDIs should take a proactive compliance approach. Conduct regular internal audits, closely track regulatory updates and implementing guidance, and maintain complete documentation to support inspections. Participation in industry associations and chambers can also help businesses stay informed and raise concerns collectively. In an evolving regulatory landscape, careful preparation and documentation remain the strongest defense under Vietnam’s stricter food safety regulation.
Avoid delays and ensure smooth approval under Decree 46/2026. Get InCorp Vietnam’s full product registration services, from dossier prep to authority submission and follow-up
Conclusion
Decree 46/2026 marks a clear shift in Vietnam’s approach to food safety, bringing higher standards and stronger enforcement. For foreign investors, this food safety regulation presents both compliance challenges and strategic opportunities. While it requires more rigorous product approvals, manufacturing controls, and documentation, it also creates a more transparent and predictable business environment for companies that invest in compliance early.
By understanding the new requirements and acting proactively, FDIs can manage regulatory risk, maintain market access, and strengthen trust with regulators and consumers. In Vietnam’s current regulatory climate, food safety is no longer just a technical obligation but a strategic priority. Companies that integrate compliance into their market strategy will be better positioned for sustainable growth under Vietnam’s evolving food safety regime.
Learn the Right Setup for Business
Expansion in the Vietnam
FAQs on Decree 46/2026
Are approvals under Decree 15/2018 still valid after Decree 46/2026?
- Not always. Decree 46/2026 may require products approved under Decree 15/2018 to be updated, reconfirmed, or re-filed under the new food safety regulation.
How long do approvals take under Decree 46/2026?
- Under Decree 46/2026, standard product declarations are reviewed within 15 working days. High-risk products require longer approval.
Does Decree 46/2026 increase import inspections?
- Yes. Decree 46/2026 applies a risk-based inspection model, meaning new or high-risk imports face more checks initially.
Is GMP mandatory under Decree 46/2026?
- Under Decree 46/2026, GMP is mandatory for health supplement manufacturing and certain high-risk products.






