Vietnam’s health product market has been booming since it opened to foreign imports in the 1990s. Currently in Vietnam over 4,000 companies are offering more than 10,000 health products. Foreign brands are dominating this sector but knowing the registration process for general health supplements requires careful attention to local regulations and procedures.
For companies looking to enter Vietnam’s general health supplement market, understanding the regulations set by the Vietnam Food Administration (VFA) is crucial. This blog will walk you through the key definitions, procedures, and regulations you need to know to register your health supplements in Vietnam. This will be helpful for the manufacturer, distributor, or investor to take the first step to bring your products to Vietnamese consumers.
Need help with Health Supplements Registration in Vietnam? Check out InCorp Vietnam’s Product Registration Services now!
What Are Health Supplements?
In Vietnam, a “health supplement” means it is a subcategory of functional food. It is meant to add to your daily diet to improve your overall health and well-being. These products may contain vitamins, minerals, herbs, or other natural ingredients.
Examples of Health Supplements in Vietnam:
- Vitamin and Mineral Supplements: They contain essential nutrients that may be missing in your diet.
- Herbal Supplements: They have plant extracts with medicinal properties.
- Probiotic Supplements: They introduce good bacteria to the gut for digestive health.
- Omega-3 Supplements: They have essential fatty acids for heart and brain health.
Market Trends of Health Supplements in Vietnam
How to Register Health Supplements in Vietnam?
Here are the key dossiers to prepare for registering health supplements:
1. Labeling:
- Clear Identification: The label must say “health supplement”.
- Nutritional Information: The label must show the amount of nutritional content, vitamins, minerals, etc.
- Disclaimer: A disclaimer “This product is not a medicine and not intended to replace it” must be stated.
- Ingredient Quantity: If the product name is the same as the main ingredient, then the label must show the amount of the ingredient.
2. Safety and Hygiene:
- Food Safety Certificate: All companies must have a “Certificate of Satisfaction on Standards for Food Hygiene and Safety”.
- Clean Facilities: Keep production, storage, and sales facilities clean and tidy.
- Employee Health: All employees who handle supplements must have regular health check-ups and food safety and hygiene training.
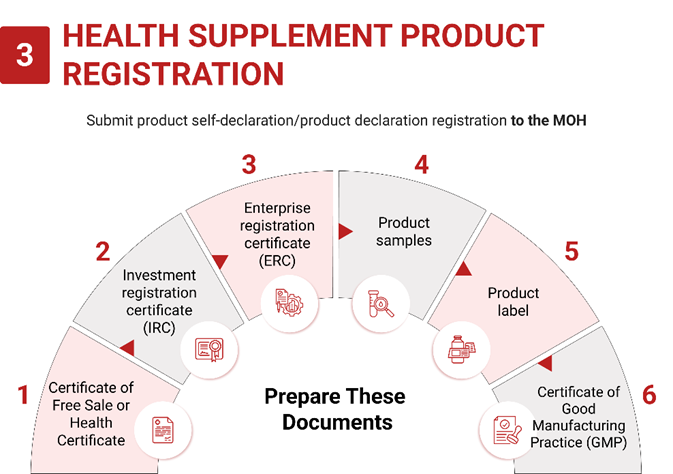
3. Application Process:
- Product Declaration: Submit product declaration (self-declaration) to the Ministry of Health (MoH).
- Registration Timeframe: 4 weeks from submission.
- MoH Certificate: Valid forever once approved.
4. Import Requirements:
- Declaration Form: Fill in the declaration form.
- Food Safety Testing Results: Original notarized copies of food safety testing results from a recognized laboratory within 12 months from submission.
- Research: Submit scientific evidence of the product or ingredient effects.
- GMP: Submit a valid Certificate of Good Manufacturing Practice (GMP) or equivalent.
How InCorp Vietnam Can Assist With Health Supplements Registration?
Vietnam’s health supplement market is expanding, but navigating regulatory requirements can be challenging. Compliance ensures product safety, prevents penalties, and secures legal approval. The process demands extensive documentation, adherence to safety standards, and government approvals, which can be complex for new entrants.
InCorp Vietnam simplifies this process by handling documentation, securing safety licenses, and ensuring regulatory compliance. Our experts manage translations, liaise with government agencies, and act as your local representative, eliminating delays and complications. Partner with InCorp Vietnam for a seamless market entry and focus on business growth while we handle compliance. Contact us today for expert assistance!

clients worldwide

professional staff

incorporated entities in 10 years

compliance transactions yearly






